Associations of C-reactive Protein with 25-hydroxyvitamin D in 24 Specific Diseases: A Cross-sectional Study from NHANES
Most diseases might be associated with acute or chronic inflammation, and the role of vitamin D in diseases has been extensively explored in recent years. Thus, we examined the associations of one of the best markers for inflammation ― C-reactive protein (CRP) with 25-hydroxyvitamin D [25(OH)D] in 24 specific diseases. We performed cross-sectional analyses among 9,809 subjects aged ≥18 years who participated in the U.S. National Health and Nutrition Examination Survey (NHANES) in 2007~2010. The generalized additive model (GAM) was used to explore the associations of CRP with 25(OH)D in different diseases, adjusted for the age, gender, examination period and race. Distributions of CRP were significantly different (P < 0.05) in gender, examination period and race, and distributions of 25(OH)D were different (P < 0.05) in the examination period and race. Generally, CRP was negatively associated with 25(OH)D for majority diseases. 25(OH)D was negatively associated with CRP generally, and the associations were disease-specific and disease category-specific. In respiratory, gastrointestinal and mental diseases, the associations tended to be approximately linear. While in metabolic diseases, the associations were nonlinear, and the slope of the nonlinear curve decreased with 25(OH)D, especially when 25(OH)D < 30 μg/L.
Similar content being viewed by others

High-sensitivity C-reactive protein could be a potential indicator of bone mineral density in adolescents aged 10–20 years
Article Open access 03 May 2022

Longitudinal changes in high sensitivity C-reactive protein associated with serum uric acid in the Korean Genome and Epidemiology Study
Article Open access 03 January 2024

Association between serum uric acid and depressive symptoms stratified by low-grade inflammation status
Article Open access 14 October 2021
Introduction
The exploration and understanding of disease mechanisms involved lots of aspects, for instance, the steady state decomposition process of metal ions was related to numerous diseases 1 , free radicals and related active substances could affect health as a mediator of tissue damage and disease 2 , defects in pre-mRNA splicing were proven to be a common pathogenic mechanism 3 , etc. In recent years, with the expanding understanding of diseases, the role of vitamin D has also been extensively explored. Vitamin D not only affected the bone metabolism regulation and female reproductive and pregnancy outcomes 4 , but also had potential correlation to cancer 5,6 . Moreover, vitamin D was also considered to have potential beneficial effects on reducing inflammation and alleviating pain 7,8 . Therefore, exploring the role of vitamin D in common diseases is interesting and meaningful.
Previous studies suggested that most diseases might be associated with acute or chronic inflammation to some extent 9,10,11 . Insufficient inflammation could lead to persistent infection of pathogens, while excessive inflammation may contribute to chronic or systemic inflammatory diseases 12 . C-reactive protein (CRP) was considered as one of the best markers for measuring inflammation caused by bacterial infection or tissue damage, which was produced by stimulation of interleukin-1 (IL-1) and interleukin-6 (IL-6) in liver 13,14 . Therefore, it is meaningful to explore the role of vitamin D in common diseases via the associations of CRP with vitamin D.
A study of 923 patients in Netherlands found that vitamin D was negatively associated with CRP in both inflammatory and non-inflammatory diseases, and the liner associations were stronger in inflammatory diseases 15 . However, a randomized placebo-controlled trial of 413 patients in Australia showed that the associations of CRP with vitamin D were not significant 16 . There is still no consensus on the association between vitamin D and CRP, and further exploration is needed. Few studies focused on the nonlinear associations between vitamin D and CRP, and vitamin D was usually considered as a categorical variable classified as deficiency or not 8,17 . However, categorical variable cannot reflect the overall distribution of vitamin D, and cannot fully explore the associations of CRP with vitamin D.
It is a challenge to explore the associations of CRP with vitamin D by the overall distribution of vitamin D rather than by the average level of vitamin D through conventional methods (e.g., logistic regression). Fortunately, the generalized additive model (GAM) had great potential in addressing associations of continues variables. We therefore applied GAM to explore the associations of CRP with 25(OH)D in 24 specific diseases based on 9,809 participants who at least had one of diseases in the 2007~2010 in the U.S. National Health and Nutrition Examination Survey (NHANES).
Results
Descriptive characteristics of subjects with the 24 specific diseases
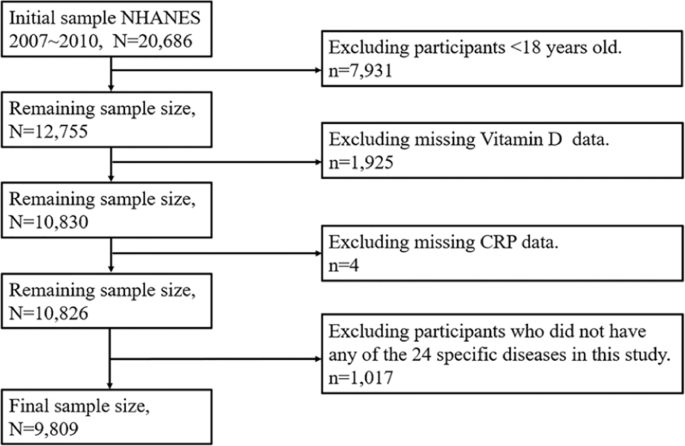
A total of 9,809 participants (4,703 males, 5,106 females) were involved in this study, and the characteristics of subjects with the 24 specific diseases were showed in Table 1, respectively.
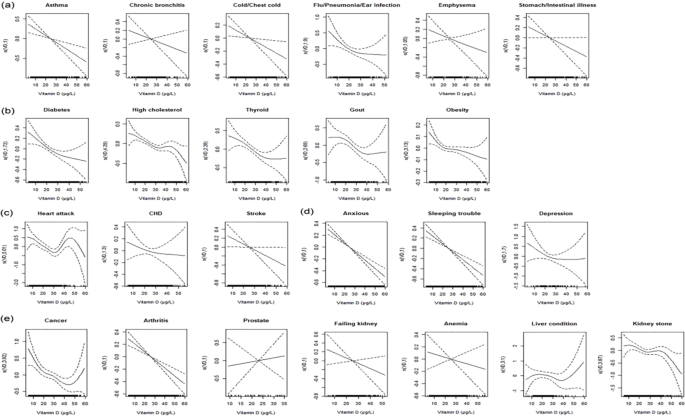
The parametric effects test of 25(OH)D in GAM
Table 3. showed the parametric effects test of 25(OH)D in GAM. Associations between CRP and 25(OH)D were statistically significant in majority diseases. Those minority diseases with nonsignificant associations may due to the small sample sizes of patients.
Data measurement
NHANES database sociodemographic information included age, gender, examination period and race. Examination period indicated the time that the participant measured the 25(OH)D, which was classified into either November 1st through April 30th or May 1st through October 31st. Because it was rather cold in the northern states in winter, the data were collected in the northern states in summer and southern states in winter 52 . Race was coded in 5 categories: Non-Hispanic Whites, Non-Hispanic Blacks, Mexican Americans, other Hispanics, and other non-Hispanic race including non-Hispanic multiracial.
During the NHANES physical examination, weight and height were measured in a standardized fashion. Height and weight values were automatically transmitted from stadiometers and scales to the Integrated Survey Information System database. This database was designed to reduce data errors and contained age- and sex-specific edit ranges for each body-size measure on the basis of previous NHANES data. If an entry was outside this range, the recorder was alerted that the value was unusual and required to verify the measurement 53 . Body mass index (BMI) was calculated by the weight divided by height squared (kg/m 2 ). Blood specimens were processed, stored and shipped to University of Washington, Seattle, WA. CRP was quantified by latex-enhanced nephelometry using a Behring Nephelometer 54 . The CDC used a standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) method traceable to measure 25(OH)D3, 25(OH)D2 and C3 epimer of 25(OH)D3. Vitamin D (variable LBXVIDMS, for NHANES 2007~2010) was defined as the sum of 25(OH)D3 and 25(OH)D2 excluded the C3 epimer of 25(OH)D3 55 .
Patients with the following diseases were defined as having been told by a doctor or other health professional: emphysema, diabetes, high cholesterol, thyroid problem, gout, kidney stone, failing kidney, anemia, heart attack, coronary heart disease (CHD), stroke, sleeping trouble, cancer, arthritis, prostate disease. Patients with asthma, chronic bronchitis and liver condition were defined as still having the disease at the time of the investigation. Patients with following diseases were defined as having these diseases started during those 30 days: cold/ chest cold, flu/ pneumonia/ ear infection, stomach or intestinal illness, anxious 56 . Patients with depression were defined as those whose Patient Health Questionnaire (PHQ-9) score ≥10, based on the participants’ symptoms over the past two weeks 57,58 . Obesity was defined those whose BMI ≥ 30 kg/m 2 59 . In addition, patients with cancer were defined that who had cancer or a malignancy of any kind.
In this study, 24 specific diseases were divided into 5 categories: a. respiratory and gastrointestinal diseases, including asthma, chronic bronchitis, cold/ chest cold, flu/ pneumonia/ ear infection, emphysema and stomach/intestinal illness; b. metabolic diseases, including diabetes, high cholesterol, thyroid problem, gout and obesity; c. cardiovascular and cerebrovascular diseases, including heart attack, CHD and stroke; d. mental diseases, including anxious, sleeping trouble and depression; e. other diseases, including cancer, arthritis, prostate disease, failing kidney, anemia, liver condition and kidney stone.
Statistical analysis
The x ± S and the M [P25, P75] were used to describe the distribution of continuous variables, and proportion was used to describe the distribution of categorical variables. Wilcoxon rank sum test was used to compare the continuous variables, and Rao-Scott-χ 2 test was used to compare the categorical variables. CRP was log-transformed to obtain normal distributions for analysis, and back-transformed for representation in the tables. Age, gender, examination period and race were adjusted to examine the associations between log-CRP and 25(OH)D. GAM was also used to explore the associations of CRP with 25(OH)D. It was a generalization of the generalized additive model (GLM), which had great potential and flexibility in addressing non-linear relationships 60 . Two variables were linear associated when the estimated degrees of freedom (EDF) in GAM was equal to one. All statistical analyses were performed by R version 3.4.3, and the package “gam” 61 , “mgcv” 62 and “glmnet” 63 were used. Statistical significance was set at P < 0.05.
Ethics approval and consent to participate
All NHANES protocols were approved by the National Center for Health Statistics’ Research Ethics Review Board, all participants signed a consent form before their participations and all research was performed in accordance with relevant guidelines/regulations. The statement of informed consent is openly available in https://www.cdc.gov/nchs/nhanes/biospecimens/participants.htm.
Informed consent
Health information collected in the NHANES is kept in strictest confidence. During the informed consent process, survey participants are assured that data collected will be used only for stated purposes and will not be disclosed or released to others without the consent of the individual or the establishment in accordance with section 308(d) of the Public Health Service Act (42 U.S.C. 242 m). Only samples from participants who have consented for future research are stored in the NHANES Biospecimen Repository and are available to researchers.
Data availability
The data that support the findings of this study are openly available in https://www.cdc.gov/nchs/nhanes/. Information from NHANES is made available through an extensive series of publications and articles in scientific and technical journals. For data users and researchers throughout the world, survey data are available on the internet and on easy-to-use CD-ROMs.
References
- Valko, M. & Jomova, K. Advances in metal-induced oxidative stress and human disease. Toxicology283, 65–87 (2011). ArticleCASPubMedGoogle Scholar
- Kehrer, J. P. & Klotz, L. O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Critical Reviews in Toxicology45, 765–798 (2015). ArticleCASPubMedGoogle Scholar
- Cooper, T. A., Wan, L. & Dreyfuss, G. RNA and Disease. Cell136, 777–793 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Norman, A. W. From vitamin D to hormone D: Fundamentals of vitamin D endocrine system essential for good health. American journal of clinical nutrition88, 491S–499S (2008). ArticleCASPubMedGoogle Scholar
- Ma, Y., Johnson, C. S. & Trump, D. L. Mechanistic Insights of Vitamin D Anticancer Effects. Vitamins and Hormones100, 395–431 (2016). ArticleCASPubMedGoogle Scholar
- Grant, W. B. Roles of Solar UVB and Vitamin D in Reducing Cancer Risk and Increasing Survival. Anticancer research36, 1357–70 (2016). CASPubMedGoogle Scholar
- Ma, R. et al. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Metab.303, E928–E935 (2012). CASGoogle Scholar
- Rai, V., Dietz, N. E., Dilisio, M. F., Radwan, M. M. & Agrawal, D. K. Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Research and Therapy18, 203 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Araki, A. & Ito, H. Diabetes mellitus and geriatric syndromes. Geriatr. Gerontol. Int.9, 105–114 (2009). ArticlePubMedGoogle Scholar
- Langhans, C. et al. Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PloS one9, e92048 (2014). ArticleADSCASPubMedPubMed CentralGoogle Scholar
- Wu, Y., Antony, S., Meitzler, J. L. & Doroshow, J. H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Letters345, 164–173 (2014). ArticleCASPubMedGoogle Scholar
- Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine21, 677–687 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kao, P. C., Shiesh, S. C. & Wu, T. J. Serum C-reactive protein as a marker for wellness assessment. Ann. Clin. Lab. Sci.36, 163–169 (2006). CASPubMedGoogle Scholar
- Bolton, C. E. et al. The CRP genotype, serum levels and lung function in men: the Caerphilly Prospective Study. Clinical Science120, 347–355 (2010). ArticleGoogle Scholar
- Kruit, A. & Zanen, P. The association between vitamin D and C-reactive protein levels in patients with inflammatory and non-inflammatory diseases. Clinical Biochemistry49, 534–537 (2016). ArticleCASPubMedGoogle Scholar
- Zheng, S. et al. Vitamin D supplementation and inflammatory and metabolic biomarkers in patients with knee osteoarthritis: post hoc analysis of a randomised controlled trial - Corrigendum. Br. J. Nutr.121, 118–119 (2019). ArticleCASPubMedGoogle Scholar
- Bellia, A. et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Internal and Emergency Medicine8, 33–40 (2013). ArticlePubMedGoogle Scholar
- Ridker, P. M. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. The American journal of cardiology92, 17–22 (2003). ArticleCASGoogle Scholar
- Khoo, A. L. et al. Translating the role of vitamin D3 in infectious diseases. Crit. Rev. Microbiol.38, 122–135 (2012). ArticleCASPubMedGoogle Scholar
- Bashir, M. et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. European Journal of Nutrition55, 1479–1489 (2016). ArticleCASPubMedGoogle Scholar
- Veldman, C. M., Cantorna, M. T. & DeLuca, H. F. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys.374, 334–338 (2000). ArticleCASPubMedGoogle Scholar
- K., M. T cell-mediated cytotoxicity in: Janeway CA, Travers JP, Walport M, Shlomchik MJ, editors. New York Garl. Sci. (2011).
- Belderbos, M. E. et al. Cord Blood Vitamin D Deficiency Is Associated With Respiratory Syncytial Virus Bronchiolitis. Pediatrics127, e1513–e1520 (2011). ArticlePubMedGoogle Scholar
- Ginde, A. A., Mansbach, J. M. & Camargo, C. A. Jr. Association between serum 25-hydroxyvitamin d level and upper respiratory tract infection in the third national health and nutrition examination survey. Archives of Internal Medicine169, 384–390 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hall, S. C., Fischer, K. D. & Agrawal, D. K. The impact of Vitamin D on asthmatic human airway smooth muscle. Expert Review of Respiratory Medicine10, 127–135 (2016). ArticleCASPubMedGoogle Scholar
- Pain, M. et al. Tissue remodelling in chronic bronchial diseases: From the epithelial to mesenchymal phenotype. European Respiratory Review23, 118–130 (2014). ArticlePubMedGoogle Scholar
- Hall, S. C. & Agrawal, D. K. Vitamin D and Bronchial Asthma: An Overview of Data From the Past 5 Years. Clinical Therapeutics39, 917–929 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Weiss, S. T. & Litonjua, A. A. Vitamin D, the Gut Microbiome, and the Hygiene Hypothesis. How Does Asthma Begin? Am. J. Respir. Crit. Care Med.191, 492–493 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Cantorna, M. T., McDaniel, K., Bora, S., Chen, J. & James, J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med.239, 1524–1530 (2014). ArticleCASGoogle Scholar
- Agmon-Levin, N., Theodor, E., Segal, R. M. & Shoenfeld, Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol.45, 256–266 (2013). ArticleCASPubMedGoogle Scholar
- Muehleisen, B. & Gallo, R. L. Vitamin D in allergic disease: Shedding light on a complex problem. Journal of Allergy and Clinical Immunology131, 324–329 (2013). ArticleCASPubMedGoogle Scholar
- Giovannucci, E. Expanding roles of vitamin d. Journal of Clinical Endocrinology & Metabolism94(2), 418 (2009). ArticleCASGoogle Scholar
- Hoogendijk, W. et al. Depression is associated with decreased 25-hydroxyvitamin d and increased parathyroid hormone levels in older adults. Archives of General Psychiatry65, 508–512 (2008). ArticleCASPubMedGoogle Scholar
- Maes, M. Major depression and activation of the inflammatory response system. Ceska a Slovenska Psychiatrie95, 233–243 (1999). Google Scholar
- Timms, P. M. et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM95, 787–96 (2002). ArticleCASPubMedGoogle Scholar
- Gagnon, C. et al. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes After 5 Years. Diabetes Care34, 1133–1138 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Karhapää, P. et al. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J. Intern. Med.268, 604–610 (2010). ArticleCASPubMedGoogle Scholar
- Krasowska, K. et al. The Preoperative Supplementation With Vitamin D Attenuated Pain Intensity and Reduced the Level of Pro-inflammatory Markers in Patients After Posterior Lumbar Interbody Fusion. Front. Pharmacol.10, 527 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Razzaghi, R. et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complications31, 766–772 (2017). ArticlePubMedGoogle Scholar
- Wei, Z. et al. Vitamin D Switches BAF Complexes to Protect β Cells. Cell173, 1135–1149.e15 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Guasch, A. et al. Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: A cross-sectional study. Cardiovascular Diabetology11 (2012).
- Amer, M. & Qayyum, R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous national health and nutrition examination survey 2001 to 2006). American Journal of Cardiology109, 226–230 (2012). ArticleCASPubMedGoogle Scholar
- García-Bailo, B. et al. Plasma Vitamin D and Biomarkers of Cardiometabolic Disease Risk in Adult Canadians, 2007–2009. Preventing Chronic Disease10 (2013).
- Kim, M., Na, W. & Sohn, C. Correlation between vitamin D and cardiovascular disease predictors in overweight and obese Koreans. Journal of Clinical Biochemistry and Nutrition 167–171, https://doi.org/10.3164/jcbn.12-81 (2013).
- McDonnell, S. L. et al. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs PLoS One13, e0199265 (2018).
- Feng, Q., Zhang, H., Dong, Z., Zhou, Y. & Ma, J. Circulating 25-hydroxyvitamin D and lung cancer risk and survival: A dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore).96, e8613 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Maalmi, H. et al. Association between Blood 25-Hydroxyvitamin D Levels and Survival in Colorectal Cancer Patients: An Updated Systematic Review and Meta-Analysis. Nutrients10 (2018).
- Mondul, A. M. et al. Vitamin D and cancer risk and mortality: State of the science, gaps, and challenges. Epidemiologic Reviews39, 28–48 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Madden, J. M., Murphy, L., Zgaga, L. & Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Research and Treatment172, 179–190 (2018). ArticleCASPubMedGoogle Scholar
- U.S. Census Bureau. Current Population Survey (CPS) - Definitions and Explanations (2008).
- Services, U. S. D. of H. and H. Poverty guidelines, research, and measurement. US Dep. Heal. Hum. Serv. website. Available online, http//aspe. hhs. gov/POVERTY/index. shtml/(accessed 16 March 2012) (2010).
- Freedman, D. M., Looker, A. C., Abnet, C. C., Linet, M. S. & Graubard, B. I. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988-2006). Cancer Res.70, 8587–8597 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- National Center for Health Statistics (NCHS) Anthropometry procedures manual. National Health and Nutrition Examination Survey (NHANES). Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures (2011).
- Huffman, F. G., Gomez, G. P. & Zarini, G. G. Metabolic syndrome and high-sensitivity C-reactive protein in Cubans. Ethnicity and Disease19, 115–120 (2009). PubMedGoogle Scholar
- Yetley, E. A. et al. NHANES Monitoring of Serum 25-Hydroxyvitamin D: A Roundtable Summary. J. Nutr.140, 2030S–2045S (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Centers for Disease Control. NHANES 2007-2008 Questionnaire Data. Available at: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2007.
- Manea, L., Gilbody, S. & McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. Cmaj184 (2012).
- Kroenke, K., Spitzer, R. L. & Williams, J. B. W. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine16, 606–613 (2001). ArticleCASPubMedPubMed CentralGoogle Scholar
- Buser, M. C., Murray, H. E. & Scinicariello, F. Age and sex differences in childhood and adulthood obesity association with phthalates: Analyses of NHANES 2007-2010. Int. J. Hyg. Environ. Health217, 687–694 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Hastie, T. & Tibshirani, R. Generalized additive models for medical research. Stat. Methods Med. Res.4, 187–196 (1995). ArticleCASPubMedGoogle Scholar
- Hastie, T. J. & Pregibon, D. Generalized linear models. In Statistical Models in S 195–247, https://doi.org/10.1201/9780203738535 (2017).
- Simon, N. et al. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology)73, 3–36 (2011). ArticleMathSciNetMATHGoogle Scholar
- Simon, N., Friedman, J., Hastie, T. & Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. Journal of Statistical Software39 (2011).
Acknowledgements
This work was supported by the Natural Science Foundation of Science and Technology Department of Jilin Province, China (grant number: 20180101129JC), Outstanding Youth Foundation of Science and Technology Department of Jilin Province, China (grant number: 20170520049JH), the National Natural Science Foundation of China (grant number: 11301213 and 11571068), the National Key Research and Development Program of China (grant number: 2016YFC1303800).
Author information
- These authors contributed equally: Fang Yang and Mengzi Sun.
Authors and Affiliations
- Department of Health Management Center, the First Hospital of Jilin University, Changchun, Jilin, 130021, China Fang Yang
- Key Laboratory of Organ Regeneration and Transplantation of Ministry of Education, School of Public Health, Jilin University, Changchun, Jilin, 130021, China Mengzi Sun, Chong Sun, Jiagen Li, Chunli Bi, Min Wang, Liyuan Pu, Yan Yao & Lina Jin
- Department of Hepatobiliary Surgery, Affiliated hospital of Beihua University, Jilin, Jilin, 132011, China Xiuning Yang
- Department of Geriatrics, the First Hospital of Jilin University, Changchun, Jilin, 130021, China Jianmeng Wang
- Department of Clinical Medicine, School of Clinical Medicine, Changchun, Jilin, 130021, China Chunxiao Wang & Meizhen Xie
- Fang Yang






